Professor John Anderson was an internationally renowned engineer and much acclaimed and inspirational figure in the world of cardiology, whose work has been highly influential around the world. He has brought great distinction to Northern Ireland and in particular to the University of Ulster. He was a founder, a director and the chief technology officer of HeartSine® Technologies Inc.

Professor Anderson formed the Northern Ireland Bio-Engineering Centre (NIBEC) and was it’s first Director. This centre is now at the centre of training of all Northern Irelands Bioengineers as well continuing to develop both pioneering research, spin-outs and technology transfer.
Professor Anderson sadly passed away in 2012.
Professor Anderson was the head of Bioengineering at the Royal Victoria Hospital in Northern Ireland, when the world’s first mobile coronary care unit was launched in 1967. Later he was responsible for the development of the world’s first portable defibrillator designed for use outside of the hospital, which was subsequently manufactured and sold throughout the world.
He has also been responsible for developing the technology utilised in many defibrillators, including semi automatic and telephone controlled devices, being marketed today. He has been instrumental in relation to the rapid development of new concepts and his technical and clinical background have led to world renowned innovations such as a new highly portable automatic defibrillator, wireless based sensor technology and numerous ECG diagnostic systems.
His role at the University of Ulster was best characterised by introducing the initial concept of a stand-alone Research Building and the construction of NIBEC was unique within Ireland.
He also pioneered much of the Universities early technology transfer ventures, with patents dating back to the mid 1980’s. He was also the Head of the School of Electrical and Mechanical Engineering at the University of Ulster (Jordanstown) for eight years. The development of companies such as Heartscape, Heartsine and more recently Intelesens with colleagues, have had immense impact on the creation of jobs, economic benefits as well as instrumental health benefits.
Professor Anderson’s areas of interest over these past 40 years have been centred around innovation related to medical electronic devices. His continuous drive has been to commercialise his ideas, to create jobs, to mentor and teach young engineers to follow in his footsteps and to advise government as to effective supporting policies. Through his business activities, and enhanced by his university, economic development and other community activities, Professor Anderson has had a major influence on several generations of young undergraduates, postgraduates, engineers and clinicians, with whom he has had contact within his role as University Professor, Head of School and company chief technical officer.
Professor Anderson was selected as “Innovation Founder of the Year 2010” by the newly formed joint university and science park venture NISP CONNECT. His academic achievements include: M Phil and D Phil in Bioengineering and a Personal Chair in Medical Electronics in 1990 by UU. In 1994 he was made a founding Fellow of the Biological Engineering Society. He was made a Chartered Scientist and a Fellow of the Royal College of Physicians. He has published over 300 papers in the field of bioengineering research and holds 40 patents in the field. He was previously an active member of the Association for the Advancement of Medical Instrumentation (AAMI) standards committees for conventional defibrillation and semi automatic defibrillation. In 2002 Professor Anderson was awarded a UK Business Fellowship, one of only twelve in the UK. During his working career, he has been responsible for ten successful start-up companies in the field of medical engineering.
His publication record has reflected his intense interest in sudden cardiac death with particular reference to the early and rapid treatment of cardiac arrest.
Contributions to sudden cardiac arrest (SCA) intervention technology have been both extensive and consistent. In fact, every AED in use today can trace its roots to technology envisioned and initially developed by HeartSine® technologists in an uninterrupted march toward better and miniaturised defibrillators. It’s a record of innovation unmatched in medical devices.
Sudden death from heart disease is a true challenge to health care. The importance of immediate care to treat sudden cardiac arrest has been well established over the past four decades. A study by McNeilly & Pemberton (under the direction of Professor Frank Pantridge, Royal Victoria Hospital, Belfast) demonstrated that a majority of heart attack deaths occurred soon after the onset of symptoms. Professor Pantridge (1916-2004) was the first to forward and implement the idea of mobile coronary care. As a result, he is known as “The Father of Emergency Medicine.”
Professor John Anderson was approached to start the Biomedical engineering group at the Royal Victoria Hospital and headed the effort to produce the world’s first mobile defibrillator to address this need. In essence, the need was to bring the expertise of the hospital to the patient to improve outcomes. The result changed the way emergency care is delivered globally, a legacy which endures to this day. This in many ways was the vision for Connected Health.
1966

The world’s first Mobile Coronary Care unit was born. Management of heart attacks outside the hospital was now possible. It soon became obvious, however, that lightweight, battery-operated defibrillators were urgently required if mobile coronary care was to become more widespread.
1969

1971
With material advances to reduce weight, other technologies were being incorporated to deliver efficacious shock therapy at reduced energies. This served to further reduce weight without compromising effectiveness. Professor Anderson et. al. developed a new waveform introduced with the PANTRIDGE PORTABLE 15 defibrillator – a unit which weighed only 15 lbs. This device was manufactured for Belfast by the Coleraine Instrument Company, and featured rechargeable ni-cad batteries, which could deliver upwards of 70 shocks. This reliable instrument became the standard of care for mobile units until 1974.
1973
John Anderson’s team now addressed a new challenge – to provide mobile continuous ECG monitoring until patient arrival at hospital, and to provide an event record for review. The system, known as CORA (Combined Oscilloscope & Recording Apparatus) was the first mobile system also to incorporate a speech channel track for rescuers to record comments, drug information, patient information, etc.
A new “fast-scan” system was also incorporated enabling a 2-hour patient record to be scanned in 5 minutes. The 3 main advantages: lightweight, continuous recording and reusable tapes. The above picture shows the new Pantridge PP15 Defibrillator in the black case, along with the new CORA unit developed for the mobile coronary care unit
Mid 1970s
The Belfast team, under Professor Anderson’s direction, were then in the forefront of mobile defibrillator technology development. Their patented portable defibrillator – the Pantridge 280 – was significant in that these new devices were a fraction of the size and weight of the first mobile unit used.
Professor Anderson is shown here with one of the early lightweight systems developed in Belfast, and subsequently manufactured for them by Cardiac Recorders of London. The unit was designed to specifications developed and prototyped at the Royal Victoria Hospital in Belfast. This unit weighed only 7.5 lbs, and became the first truly lightweight portable defibrillator for emergency services. Six hundred units were sold in the United States alone.
The concept of telephone-controlled defibrillation was suggested in the early 1980s (Buessman, 1982). The technology was progressing towards having systems available for automatic external use in public areas, and this device enabled users to connect patients to electrodes that were monitored by clinical experts at a base station through a regular telephone line. The patient unit automatically dialled the base station, and retrieved any patient medical records that could be available. ECG could thus be monitored remotely. This leading-edge device could then send a control signal back over the telephone to defibrillate the patient! The rescuer could therefore use this device remotely with minimal training, having the support of a skilled operator controlling therapy.
The Belfast experience, as it came to be known, became an emergency care model that was quickly adopted throughout the western world. Mobile coronary units were modelled on the Belfast plan, and first adapted for use throughout the United States, Britain, Switzerland, Norway, Holland, Australia, Japan, and Brazil. According to Richard Crampton (MD, FACP, FACC, Prof. of Medicine, U. of VA), the introduction of the mini-defibrillator has been one of the most important contributions, (“The Acute Coronary Attack,” J.Pantridge, J. Adgey, J. Geddes, S.Webb, foreword, 1975).
Professor Anderson and his group spearheaded design advancements that are used in current defibrillator models worldwide. A host of patents followed. Some of the “firsts” that came out of the Belfast group include:
John Anderson founded HeartSine in 1998 in conjunction with a group of investors to further the development of portable defibrillators based on his early Belfast experience. While lightweight defibrillators were providing the type of instruments required by medical personnel, there was a clear need to develop defibrillators which could save lives by non-professional users with minimal training.
The first HeartSine defibrillator was the AED – The Automated External Defibrillator. The AED was introduced with LCD screen for ECG trace and graphic (with written) instructions. Audible prompts also helped coach users in proper operation of the unit. A professional model (SAM-001) was introduced, with manual shock override control, and ECG screen. A step-down unit was soon released removing manual override (SAM 002) and a third variant (SAM 003) followed with only Icon display (no ECG) and no manual override. This was HeartSine’s first direct foray into addressing the growing need for easy-to-use products for public access defibrillation. Since professional responders were experiencing improvements in outcomes, a natural progression would be to make these instruments easier to operate for laypersons with only basic training. Today, these units are becoming commonplace in shopping centres, airports, gyms and health clubs, schools, and many other public access areas.
HeartSine samaritan® PAD
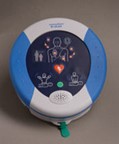
Samaritan PAD with CPR Advisor (currently not available in the U.S.)
Recognising the importance of CPR with AED use, the samaritan PAD also provides rate coaching for chest compressions. Additionally, HeartSine has developed a unique method for accessing CPR performance. The HeartSine samaritan PAD with CPR advisor — 500P — uses an Impedance Cardiogram (ICG) to determine if CPR is being applied hard enough and at the correct rate.
New research is ongoing for product improvements, with the goal of providing new and innovative technologies for next-generation lifesaving devices accessible to everyone.
As well as the developing mobile coronary story and another parallel process was developing. Professors Anderson, McLaughlin and McAdams have a 25 year history of developing successful patent exploitation in the area of medical sensors and electro-stimulation devices, following the impact that Pantridge, Adgey and Anderson had on mobile coronary care in Northern Ireland.
This more recent work has been commercialised to companies such as Heartsine Inc., Intelesens Ltd., Heartscape Inc. (sold to Verathron 2010), Tyco (licensed the biggest selling disposable ecg electrode), SHL Telemedicine, Phillips and Air Products.
These companies’ products have now been well established and include the world’s best selling disposable ecg electrode, telemedicine based 12 -lead electrodes, the most compact AED and a smart wireless chest based ecg, respiration rate, temperature and SpO2 monitoring. The impact is now at a global level and all these three connected health spin-out companies have global sales that impact on both well-being of patients and economic savings especially related to patient care.
Heartscape developed out of an initial spin out from the University of Ulster called NIRAD. It was set up by Professor John Anderson and Mr Andrew O’Hare and developed telemedicine, ecg mapping and medical device capability. The company was initialled formed as NIRAD, then Brunswick, Meridian Medical and more recently Heartscape.
Under Meridian Medical Technologies the company focused on serving the emergency and early intervention healthcare markets. Meridian was comprised of two business units: Cardiopulmonary Diagnostics and Specialty Pharmaceuticals. Meridian’s main product in Cardiopulmonary Diagnostics was the Prime ECG™ electrocardiac mapping system, a non-invasive device designed to detect a heart attack earlier and more effectively than current technology.
Meridian’s Specialty Pharmaceuticals’ manufactured the life-saving EpiPen® auto-injector, and was the sole supplier of auto-injectors for nerve agent antidotes to the U.S. government and other NATO allies, states and local municipalities, and manages a large commercial drug delivery business.
The company also developed telemonoitoring devices, which were licensed from NIBEC (remote single–lead. 12 lead- ecg, bp, weight and SpO2) for the connected health market as far back as 1985. These technologies were further licensed or sold to selling SHL Telemedicine (Israel) and at one stage Phillips.
The company also licensed an important patent developed by Anderson, McLaughlin and McAdams to Tyco International Ltd. (initially Ludlow Medical) which is still used in the mass production of the world’s best selling diagnostic ecg electrode.
Key patents, screen printing electrode technology and the venture into Connected Health in the late 1980’s were to lead Anderson, McLaughlin and McAdams to set up a new venture in the late nineties that was initially called Sensor Technology and Devices Ltd. And later became known as Intelesens.
Intelesens is an established growth company developing and marketing advanced wireless, body worn, vital signs monitoring devices, medical sensors and electrodes for use primarily in Remote Patient Monitoring (RPM), and Personal TeleHealth applications.
Intelesens began operations in 2001 as Sensor Technology & Devices Ltd, a spinout company from NIBEC at the University of Ulster in Northern Ireland, having specific expertise in the application of sensor technologies to many aspects of physiological measurement. The three founders, Anderson, McLaughlin and McAdams were to join forces for the first time and combine many years of medical electronics, sensors and electrodes experience.
The Company established a recognised leadership position within the medical sensor industry based on its physiological sensor design and development capabilities and unique manufacturing processes for:
- Defibrillation pads
- ECG, EEG, EMG, screen printed electrodes
- “Electronic Nose” sensing for trace gas detection
- Pulse Wave Velocity sensors
- Respiration sensors
- Optical sensors
The design of customised sensors is one of the Company’s fundamental core competencies and current sources of revenue. In its early years the Company focused exclusively on contract development of disposable sensors typically for OEM applications in the biomedical industry. The Company’s sensor manufacturing process, which allows the electrodes/sensors to be screen-printed onto stabilised polyester substrate sheets with a three-layered composition of carbon, silver + dielectric inks, is both unique and proprietary.
In more recent years the focus of the Company has expanded to include the design and development of wireless vital signs sensors and devices for use in remote patient monitoring and telehealth applications. The devices no utilise integrated wireless systems, pattern recognition software and smart analysis systems. In 2009, the Company changed its name from Sensor Technologies & Devices Ltd to Intelesens Ltd to more accurately describe this new direction and innovative nature of the business.
Intelesens maintains a strong academic–industrial collaboration and close working relationship with the Nanotechnology and Integrated Bioengineering Centre (NIBEC). This relationship greatly enhances the technical expertise and overall capabilities of the business.
Intelesens is attractively positioned to benefit from the expected flood of spending aimed at reducing the overall cost of healthcare. Remote patient monitoring is one area where Intelesens stands to capitalise due to its unique and superior product solution. Intelesens is developing a portfolio of products that will deliver improved quality-of-care to patients and help to significantly reduce healthcare costs by lowering the need for hospitalizations and outpatient visits.
Intelesens began selling its first wireless based product, a CE approved remote cardiac event monitoring (REM) device, in 2008. This 4-algorithm, wireless, chest-based Cardiac Arrhythmia Detection Monitor, which enables physicians to diagnose and effectively manage treatment for patients with cardiovascular disease, is currently being sold under the V-Patch brand name through an OEM Channel in the EU and China.
During Q2 2009, the Company released a “next generation” 10-algorithm wireless Cardiac Arrhythmia Detection Monitor that will be sold, initially in the EU, through the same OEM as well as other potential OEM channels. In addition, the Company designs and sells specialty medical sensor products on an OEM basis to a variety of medical companies.
In 2010 Intelesens won the prestigious Most Promising Technology Award at the 4th Annual Silicon Valley Technology Leaders Awards in San Jose, California and the same year received formal confirmation that their wearable wireless hospital monitor, Aingeal, has been awarded class 2 regulatory approval by the FDA authorities in the USA. Following the completion of successful clinical trials in Massachusetts General Hospital in Boston in 2009, FDA clearance now enables the company to deploy Aingeal into US hospitals and healthcare organisations. Aingeal received its European CE class 2 approval in 2009.
The Aingeal device enables patients to be monitored, continuously and wirelessly (Wi-Fi), from the moment they arrive in a hospital until the time they are discharged. Where currently monitored patients are restricted to their hospital beds, the Aingeal body worn monitor allows free movement, promoting physical activity and recovery. The Aingeal device is unique in that it measures the patient’s ecg, heart rate, respiration-rate, temperature and motion and sends that information wirelessly so that it is immediately and easily accessible by nursing and other medical staff. For the first time however, this device also measures the patient’s respiration rate (early warning vital sign). Clinicians can access the patient data through any web browser freeing them from paper charts and records and reducing the costs of taking routine observations.
The Nanotechnology and Integrated Bioengineering Centre (the new NIBEC) has evolved from early electro-physiology research conducted by Prof John Anderson some 25 years ago. The original Northern Ireland Bioengineering Centre was formed in 1990 under the Directorship of Professor Anderson with a strong emphasis on sensors; biomaterials and device miniaturisation.
Dr Christiaan Barnard, the South African surgeon who conducted the first heart transplant operation, opened NIBEC’s new EU-funded building in 1994 and in 2004, Nobel Laureate Professor Ivar Giaever opened the new Nanotechnology wing.
In recent years the theme of nanotechnology has underpinned much of the work and this led to the opening of a new twin-building in 2003. In 2006 these two research themes combined and the current Nanotechnology and Integrated Bioengineering Centre (new NIBEC) was launched.
A number of research groupings such as the BEST Centre; NanotecNI; NICAM and most recently MATCH and CHIC are examples of alliances that are housed within the NIBEC structure.
During the last 25 years this valuable work has continued and many innovative devices and systems have evolved at NIBEC, particularly in the fields of defibrillation, connected health, body surface mapping, nanotechnology, tissue engineering, sensors, point of care monitoring and implantable cardiovascular devices.
RVH Links: A collegial grouping of staff between NIBEC and the Royal Victoria Hospital , Belfast (and other Trusts) now exists (CACR) with strengths in fundamental physiology, cardiovascular modelling, material science and clinical practice in the provision of a focused vehicle to inculcate best scientific practice into the clinical setting.
NIBEC at Present: The Centre, managed by Professor Jim McLaughlin since 2001, today hosts ten research groups with over eighty researchers. The facilities cover over 2000 sq. ft. and boasts some of the world’s finest instrumentation. A much stronger emphasis is now on developing a basic understanding of nanomaterials and how this technology can be applied to medical devices. Themes such as Connected Health, Tissue Engineering and Clean Technology and Nanomaterials are dominating activities. The centre has been successful in the area of technology transfer with numerous filed patents and a stream of successful spin-out ventures such as Intelesens (formerly ST&D Ltd), Heartsine, Heartscape and a new spin-in SiSAF. More recently the newly formed umbrella a research institute (Engineering Research Institute) has seen further spin outs such as Axis Composites and Lenis Aer
NIBEC’s fundamental materials research has been directed towards, and translated into, a range of advanced commercialised products through technology licensing and, more recently, via a number of Connected Health spin-out ventures. The centre since it’s opening has received over £40M of funding, graduated over 200 PhD students and 220 Biomedical Engineers (course started in 2001).
Key themes in NIBEC current research are:
- Sensors and Connected Health
- Tissue Engineering and Regenerative Medicine
- Clean Technology
- Nanomaterials
This work has led to over thirty-five patents being filed and 3 Connected Health spin-out companies (over 150 employees). The centre has developed and licensed numerous devices such as PRIME ECG, Vital Signs Wireless Patch, 12-lead holter-telemonitoring, AED defibrillators, telemonitoring platforms, the world’s biggest selling ECG electrode and a range of licensed algorithms to companies such as Heartsine, Intelesens, Samsung, Tyco and Meridian Medical. New development include imepediometric point of care devices, tissue engineering structures, bioactive coatings, e-nose systems, drug eluding polymers, smart catheters/stents and nanoparticle fabrication and characterisation.
These developments have culminated in the award of a new £5M Competence Centre called the Connected Health Innovation Centre (CHIC), which will focus on industry-led research, underpinned by the University’s research capability in this area. McLaughlin has used the success of Intelesens to take up a leadership role with the European Connected Health Campus (80 members including Intel and Bosch) which is both impacting on NI Government and Global Business Policy.
The products associated with the NIBEC designs have already impacted in the following way:
- Stimulated world-wide interest in the possibility of home and hospital based wireless monitoring
- Created over 120 jobs across all 3 Connected Health spin-outs
- Saved lives, aided the elderly, improved the quality of life and cut costs.
- Developing new funding models involving multi-national companies
- The Technologies are used by the Wellcome Trust, Government, CIMIT (Boston) and various companies as exemplars of the future of healthcare
- The concept of Connected Health in Northern Ireland was derived from much of this early work and NIBEC & Intelesens have led and inspired many initiatives such as the BEST Centre, MATRIX Life and Health Sciences (2008-2011), ECHCampus (2009-) and now the European Connected Health Alliance (2011-) culminating with NIMAC which will see a joint agreement between Finland, ROI, Northern Ireland, the Manchester Eco System and Catalonia as well as the Northern Ireland DOH & DETI signing a strategic and joint Connected Health MOU
- The Founders of these NIBEC spin outs are invited globally to talk about the Innovation Process with talks presented in Japan, Finland, UK (IOP, TSB, RSC, IET, RAE), and the ROI (RIA).
Close tri-lateral partnerships with industry (local and international) and clinicians will establish further market and clinical roadmaps for each product family along with field testing and rapid feedback.
